Water Systems
Water
Water is life. Next to oxygen it is the most crucial substance for us to survive. But not just any water will do, it has to be fresh water. Ocean water (salt water) is not consumable by us, it is too salty. We in Canada are absolutely swimming (pardon the pun) in potable (means drinkable) fresh water. The Great Lakes (which we share with the US) carry 20% of the world’s fresh water supply. Not having access to potable water is a major issue in many parts of the world and many millions of people must spend hours every day retrieving drinkable water every day from far away sources, often across dangerous terrain.
Earth’s Supply of Water
Water is abundant on our planet. Roughly 2/3 to 3/4 of the surface of our planet is covered with it but most is salt water. Only about 3% of the world’s water is fresh water and most of that is locked up as ice or moves below the surface of our planet as ground water or in underground aquifers. In fact only about 1% of the fresh water supply exists in lakes, rivers, wetlands and gaseous water or soil moisture. This means that only 1% of 3% fresh water of the world is accessible to us in some more or less direct way.
Most of Canada’s fresh water is actually to be found underground as ground water. As precipitation hits the soil it soaks through until it reaches a layer that does not allow it to pass. The water fills the spaces between the soil particles and near the impermeable layer it accumulates to a degree that it fills all of the available space. The uppermost part of this so called zone of saturation, also called the groundwater zone is called the water table. Most rural homes access this water in the form of wells. Most often the material that forms the impermeable layer is made of rock or hardened clay. Sometimes the structure of these underground layers is such that it allows for permanent reservoirs of water to accumulate that get replenished through precipitation. Such reservoirs are called aquifers.
Most of the Earth’s fresh water is locked up in ice in the form of polar ice and glaciers. It is estimated that glaciers and ice sheets contain more than 40 million cubic kilometers of frozen fresh water. The Antarctic Ice Sheet at many points is more than 2 kilometers thick.
The Cycling of Water
Water on Earth is constantly changing from one state into another. It is estimated that 5 of every 100,000 liters of Earth’s water is changing state in the time it takes for you to read this paragraph. Somewhere water is freezing, thawing, evaporating or condensing.
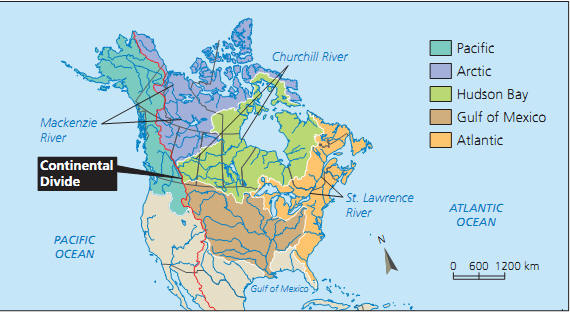
Water that falls to earth in the form of precipitation slowly returns to the large bodies of water on our planet, most likely an ocean. Due to geographic features, large areas of land end up emptying into the same body of water. Such areas are called watersheds. Here’s a map of the watersheds of Canada:
Water’s Influence on Weather and Climate
Consider two places in Canada: Timmins, Ontario and Victoria BC.
The average snowfall in Timmins is over 300 cm compared to less than 30 cm in Victoria and the average temperatures in Timmins range from 17oC in July to -18oC in January compared to 16oC in July to 5oC in January in Victoria. These two cities sit nearly at the same Latitude (about 48.4 N) but have significantly different climates and weather. Why should this be?
The answer is simple, Victoria sits near, actually within, a massive body of water (the Pacific Ocean) and Timmins does not. Water has a massive ability to absorb and release heat energy due to its many hydrogen bonds (never mind what a hydrogen bond is, look it up if you feel like it 🙂 ) The ability to absorb heat energy compared to how much the temperature of a substance increases is called the Heat Capacity. Water has a massive heat capacity, which means it takes a lot of energy to heat it up for each degree and when it cools down, it releases this energy to the outside.
This means that in the warm season, the surrounding water absorbs a massive amount of heat energy that is slowly released to the surrounding atmosphere during the cold season, keeping the climate moderate.
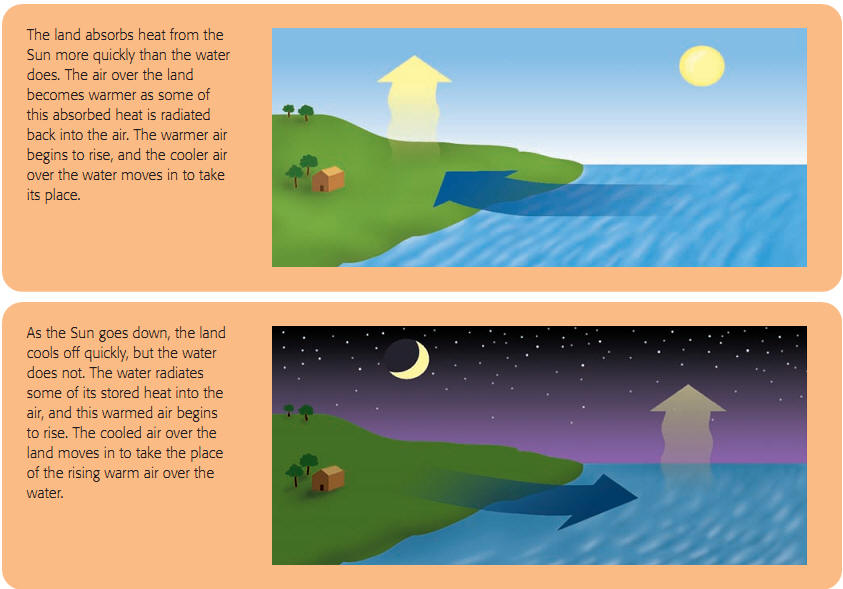
Since soil is darker than water, it absorbs radiant heat faster than neighbouring water. During the daytime, this means that the ground heats up faster and the air above it rises faster than over the water. This creates a wind from large bodies of water towards the land during the daytime. At night the trend is reversed when the ground cools off faster than the water and the water continues to heat the air above it, creating a wind from the land to the water.
Ice Sheets
Part of what keeps our climate stable is the interplay between how much radiant heat from the sun is absorbed by the surface of our planet, how much is radiated back to space and how much is again re-reflected back by our atmosphere and gasses contained in it (like carbon dioxide, methane and water).
Ice is more reflective than liquid water, so a change in the amount of surface that is covered in ice has an effect on how much heat is absorbed and how much is reflected back to space. Shrinking ice sheets would make room for either liquid water or underlying soil/rock both of which are less reflective and absorb much more heat.
Water Quality/ Sewage and Water Treatment Methods
Water is an excellent solvent for a great variety of substances but that means that it is also very “vulnerable” to contamination by materials that are undesirable for us to consume.
Biological contaminants like bacteria, fungi and viruses, chemical contaminants like pesticides, pharmaceuticals, road salt and industrial compounds, and physical contaminants like sand, animal waste, plant debris etc. are all unhealthy or at least not pleasant to consume.
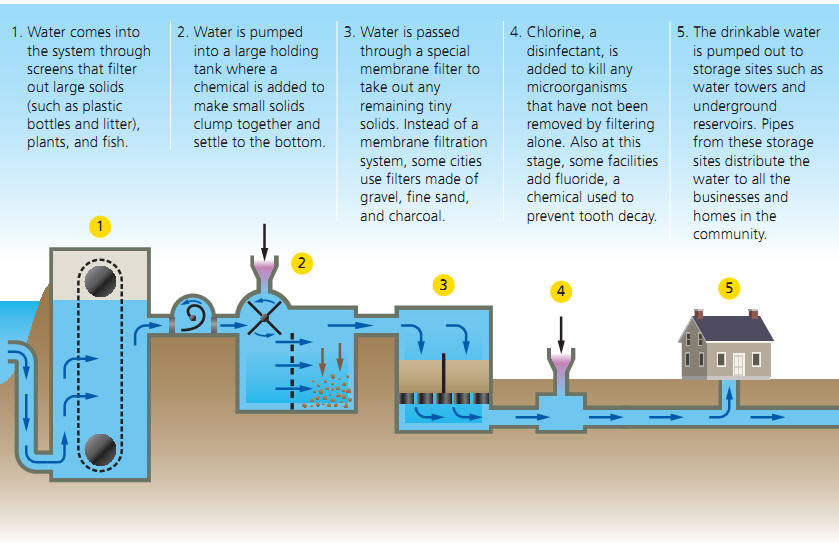
In order for water to be made fit for us to consume we must reduce such contaminants to non-threatening levels. Removing them 100% is often impossible but also unnecessary. The basic process consists of a variety of filtering methods, including the addition of coagulants (substances that promote the clumping of small particles) and the use of filters that capture dissolved substances such as activated charcoal and the addition of disinfectants or the use of UV light to kill of micro-organisms.
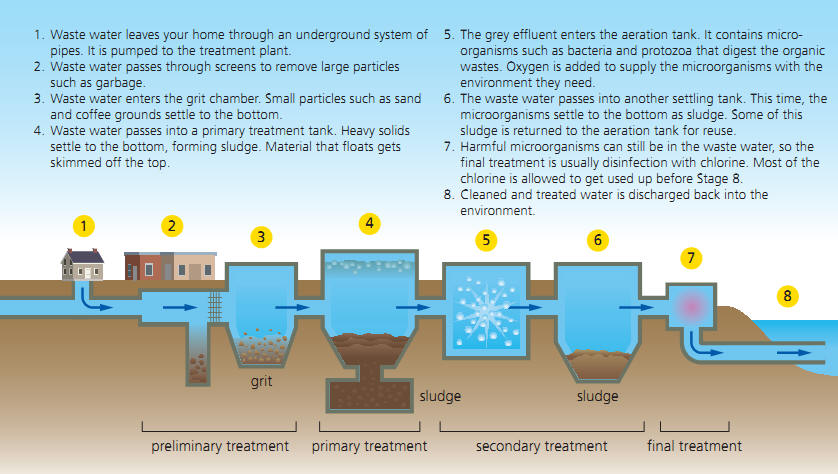
Sewage treatment is on a basic level not that much different but uses a much more contaminated “input” substance (obviously) and produces not potable (drinkable) water but water that has been decontaminated only to such a degree that the release into nature no longer presents and obvious health hazard. Natural degradation processes complete the process after release.
Both processes are expensive and the less contamination we all deliver into this system the less our municipalities need to invest in keep our access to clean drinking water secure. Clean drinking water is one of the most underappreciated benefits of modern life. Contaminated drinking water is one of the largest preventable killers of humans on the planet. Estimates rank somewhere in the 3-4 million a year. Another frightening statistic is that every 20 seconds a child dies from a disease it caught from contaminated water. This means that during one of our 30 minute lessons 90 children have died.